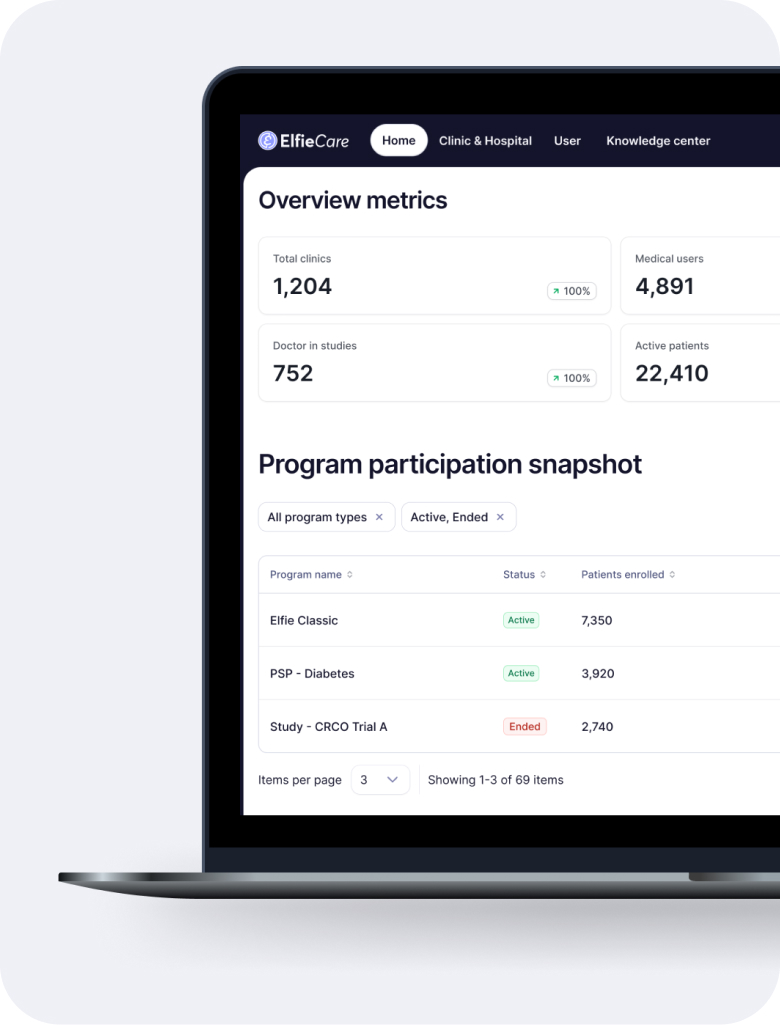
One platform for all
Run patient programs and trials to generate real-world evidence and adherence.

Deliver results in a few weeks, with compliance at heart.
With experience in 30+ countries, Elfie is a true global partner to deliver impact in compliance with local clinical, data, pharma and consumer regulation.
CLINICAL TRIALS
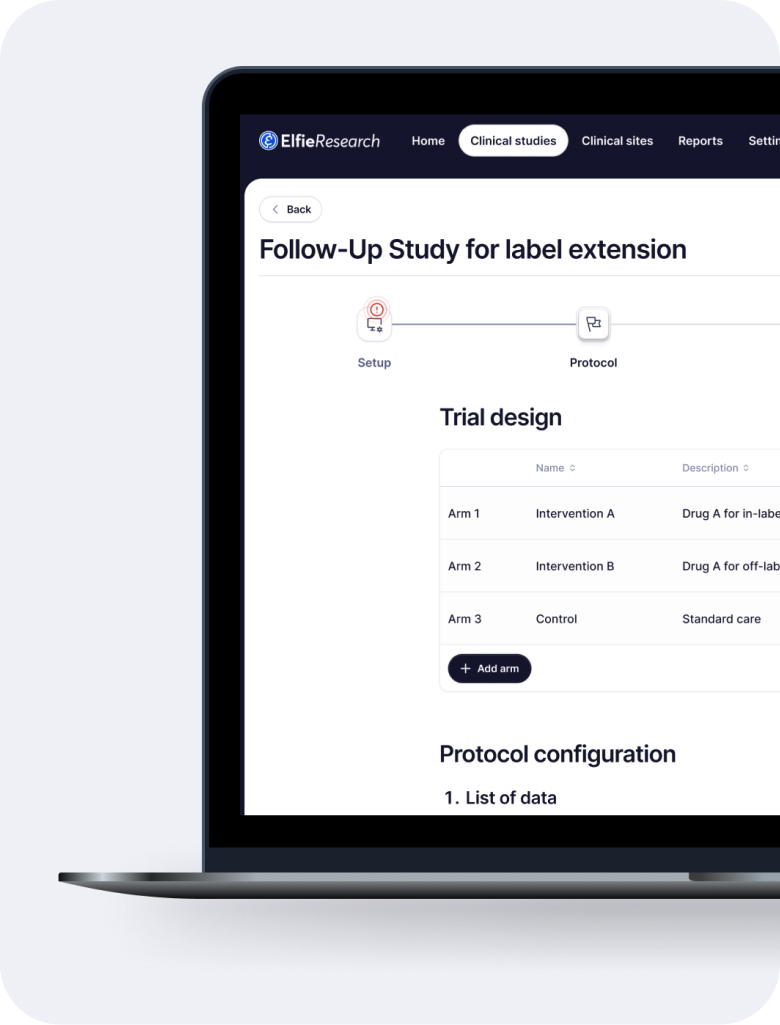
Cost-efficient decentralized and hybrid trials
ElfieResearch specialized in running trials and medical studies in markets where historically the unit economics didn’t work out.

Decentralized trials

Observational studies

Global e-consent and compliance

Participant- & investigator-first design
AWARENESS CAMPAIGNS
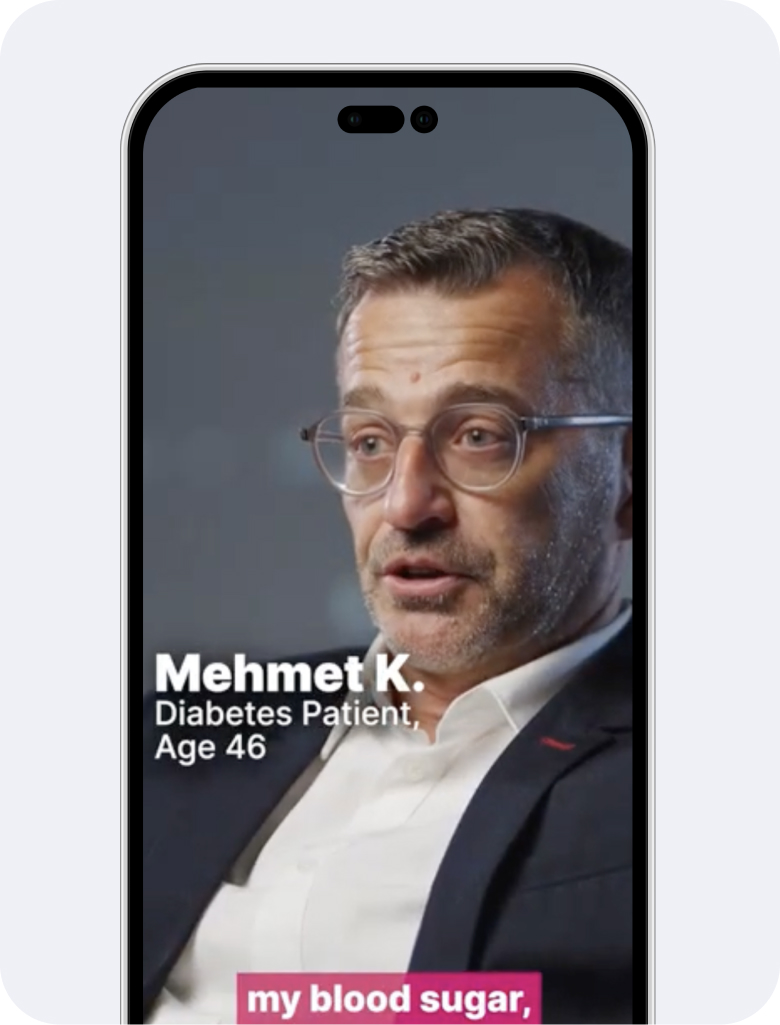
Increase awareness and adherence
Elfie conducts campaigns that drive disease awareness and medication adherence generating a measurable ROI based on sales uplift.

Go-live within two weeks globally

Real-time reporting

KOL, doctor and patient content

Medical impact and ePRO
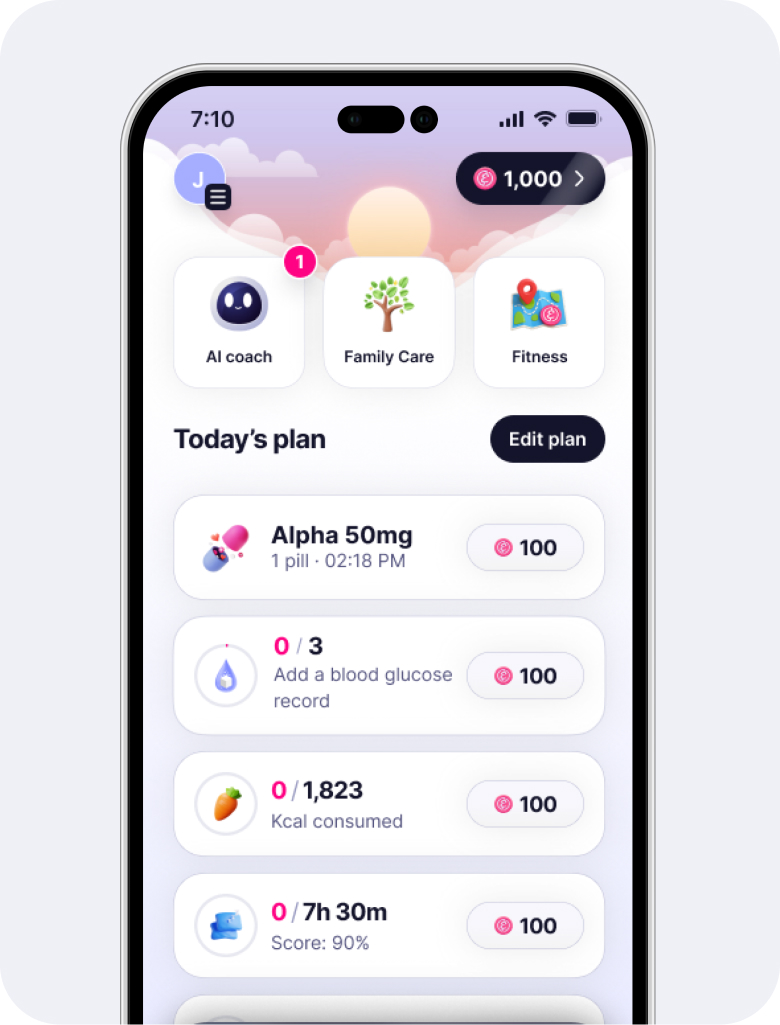
Take a quick tour of the Elfie app
GET BETTER
Partner with Elfie, today.